Degree of Dissociation Formula
What is the general formula for alkenes. A typical C-C single bond has a dissociation.

Calculate The Percentage Degree Of Dissociation Of An Electrolyte Xy 2 Normal Molar M Youtube
Visit BYJUS for detailed information.

. If you do not have a water analysis you can use. Acidity is defined as a pH of less than 7. On the calculation of the degree of saturation of seawater with respect to calcium carbonate under in situ conditions.
Eliot in full Thomas Stearns Eliot born September 26 1888 St. To calculate unsaturation 1 degree of saturation equals _____ hydrogen atoms which means a ring or a double bond each subtract _____ hydrogen atoms from the max. To find pH for a given molarity you need to know how to work with logarithmic equations and a pH formula.
Divided by the number of formula units initially dissolved in solution and means the number of particles per formula unit of the solute when a solution is dilute. This calculator converts automatically the pressure to bar with the following conversion factor. However a free Theoretical Yield Calculator is used for chemical reactions that compute the theoretical yield according to the theoretical yield formula.
Kp and Kc are the equilibrium constants for an ideal gaseous mixture. Select two compounds above and this calculator will predict whether or not the reaction will occur in waterThis is simply based on the solubility chart of inorganic compounds. With molecular formula CH3OH has a molar heat capacity C p of 811 Jmol K.
Eliot exercised a strong influence on Anglo-American culture. The pH scale ranges from 0 to 14 under usual conditions and measures the acidity of an aqueous solution. The obtained osmotic pressure with formula 2 is in psi pounds per square inch.
It is a tribasic acid. In reality the answer will be slightly different. Examples of Crystalline Structure.
The pH scale which normally spans from 0 to 14 in water is used to determine the pH of an aqueous solution. This is derived from the molarity of protons hydrogen ions or H in the solution. It is present in teeth and bone and helps in metabolic processes.
Louis Missouri USdied January 4 1965 London England American-English poet playwright literary critic and editor a leader of the Modernist movement in poetry in such works as The Waste Land 1922 and Four Quartets 1943. This is case of strong acid titrated with strong base so we expect pH at equivalence point to be that of neutral solution - that is 700. PKa the negative logarithm of the acid dissociation constant molecular structures molar weights density and melting and boiling.
1 psi 6894810-2 bar. Here we will study the pH value formula and how pH value is calculated in detail. Heat Capacity - C - is a characteristic of an object - the amount of heat required to change its temperature by one degree.
The degree of dissociation is the fraction of the original solute molecules that have dissociated. The crystalline structure of carbon is an age-old example and illustration of how the arrangement of atoms defines the properties of a crystal. A double replacement reaction will occur if a formation of a precipitate gas or water takes place.
First sulfuric acid has pK a1 -3 very strong acid but second dissociation step has pK a2 20 so it is much weaker. To learn about its Structure Formula Synthesis Properties Uses and FAQs of orthophosphoric acid. Three reasons for that.
This compound is a _____ alkyne. A 20 ml portion of this solution requires 15 ml of 012 M NaOH solution to reach the titrations equivalence point. This compound is a _____ alkyne.
Dissolve 20 g of the unknown monobasic acid sample in 100 ml of water. Orthophosphoric Acid H3PO4 Phosphoric Acid - Orthophosphoric acid H3PO4 is one of the most widely used chemicals. Geochemica et Cosmochemica Acta 341261-1291.
A pH of more than 7 is classified as basic. Goyet C and A. When equilibrium concentrations are represented in atmospheric pressure Kp is the equilibrium constant to use and when equilibrium concentrations are expressed in molarity Kc is the equilibrium constant that should be used.
The equilibrium constant denoted as k is a number. New determination of carbonic acid dissociation constants in seawater as a function of temperature and. A pH of 7 is regarded as neutral.
For dissociation in the absence of association the van t Hoff factor is.

Calculate The Degree Of Dissociation Of Hi At 450 C If The Equilibrium Constant For The Diss Youtube

A Calculate The Degree Of Dissociation Of 0 0024 M Acetic Acid If Conductivity Of This Solution Youtube
Degree Of Dissociation Pka Of Weak Acid Calistry
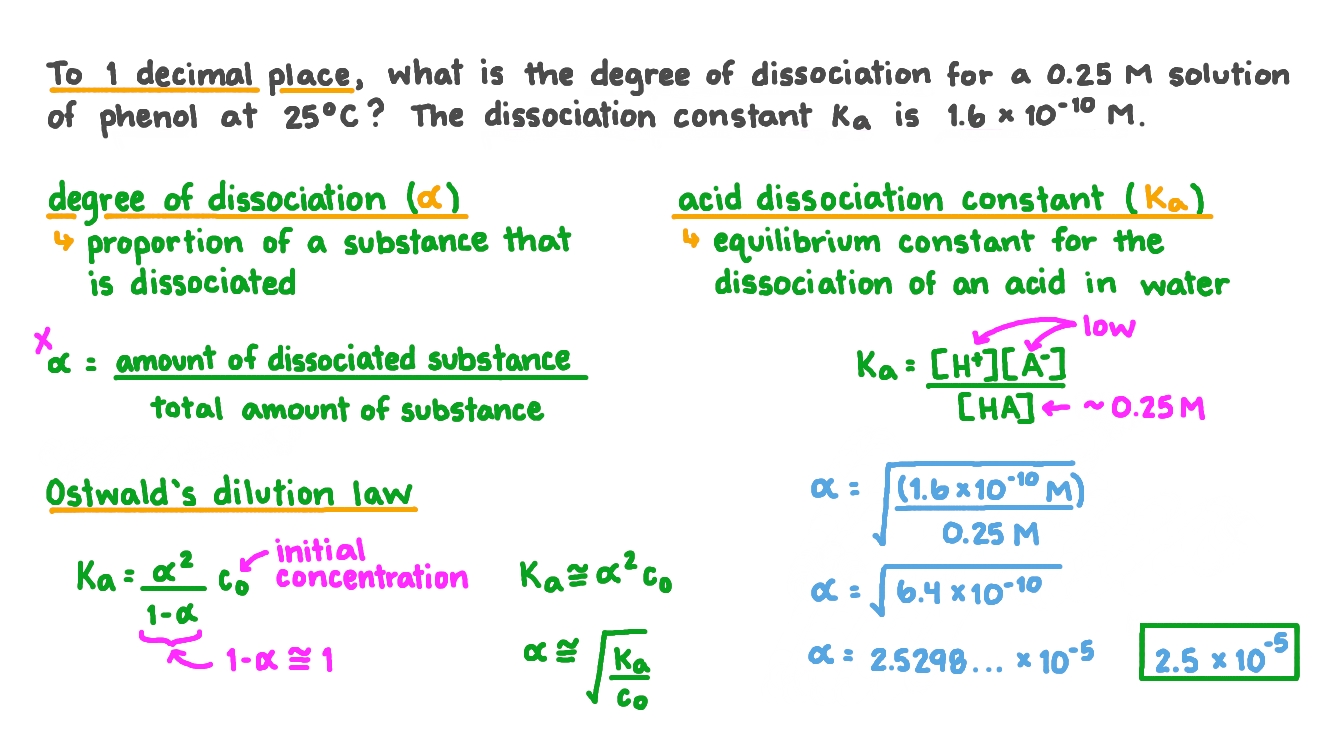
Question Video Calculating The Degree Of Dissociation Of A Solution Of Phenol Given The Acid Dissociation Constant Nagwa
No comments for "Degree of Dissociation Formula"
Post a Comment